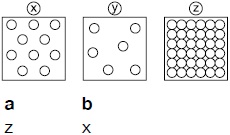
Subject :NSO Class : Class 5
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 4
Ans 1:
Class : Class 4
b and d are because of transpiration a is evaporation, and b is correct it is because of precipitation
Ans 4:
Class : Class 3
Shreshta the ans is not b coz in b precipitation is not happening condesation is happening that is why ans is c
Ans 8:
Class : Class 3
I'm a simple girl who loves having fun chatting and meeting new people. You will always find me smiling and happy because nothing disturb me!
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Class : Class 6
Our body is made of mostly made of impure water which is a good conductor of electricity. Our body also has a lot of metallic minerals like iron which conduct electricity.
Ans 2:
Class : Class 6
because our body contains minerals like iron and has a large quantity of un - distilled water
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Class : Class 6
because our body contains minerals like iron and has a large quantity of un - distilled water
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Class : Class 6
because our body contains minerals like iron and has a large quantity of un - distilled water
Post Your Answer
Subject :NSO Class : Class 6
Ans 2:
Class : Class 6
Answer (C) is correct because our body is a good conductor of electricity and it has even been mentioned in the synopsis. Now, that means that (i) is correct so automatically B and D get canceled. The air in no form acts as a conductor and is an insulator. So C is also canceled. This leaves us with our final aswer A.
Ans 3:
Class : Class 6
actually our human body consists 70% of water and if we touch electricity they are conducted through our body to either to another person or to any metal or to directly the ground.
Ans 5:
Class : Class 6
because our body contains minerals like iron and has a large quantity of un - distilled water
Post Your Answer
Subject :NSO Class : Class 6
Ans 3:
Class : Class 3
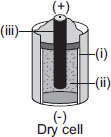
All answers are wrong - even 'D' and 'C'. 'C' would, however, be right if the line for '(ii)' was a little shorter.
Ans 19:
Class : Class 10
c is the only correct ans because in a zinc carbon dry cell zinc container is the negative terminal of the cell and carbon is the positive terminal of the cell and we need a electrolyte to convert chemical energy into electrical energy i.e. ammonium chloride.
Post Your Answer
Subject :NSO Class : Class 6