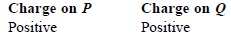Subject :NSO Class : Class 8
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
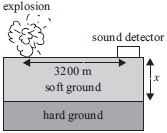
Subject :NSO Class : Class 8
Post Your Answer
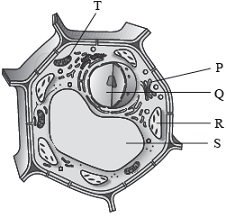
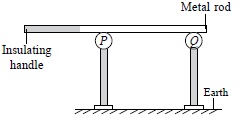
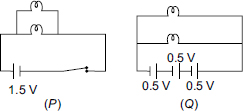
Subject :NSO Class : Class 8
Ans 8:
Class : Class 8
The correct answer is A as sphere P will acquire a positive charge due to conduction. This is also written in the solution.
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Post Your Answer
Subject :NSO Class : Class 7
Ans 5:
Ans 6:
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 6
Three groups of materials are given below :
I : Salt, sugar, flour
II : Copper, gold, silver
III : Petrol, kerosene, paper
Identify the property common to each group.
| I | II | III | |
| A | Solid | Bad conductors of heat | Liquid |
| B | Edible | Non-metallic | Non-inflammable |
| C | Edible | Metallic | Inflammable |
| D | Solid | Good conductors of heat | Non-inflammable |