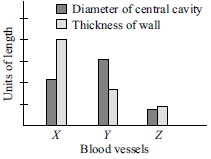
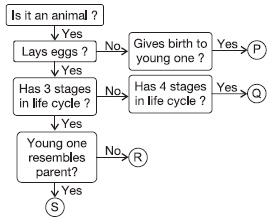
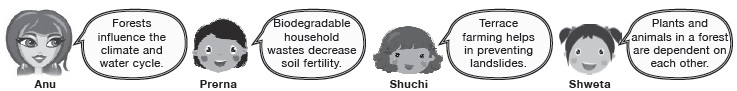
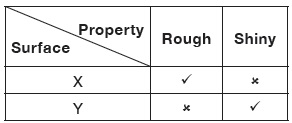
Subject :NSO Class : Class 7
Subject :NSO Class : Class 4
Ans 1: (Master Answer)
Class : Class 1
B
Q is an animal that lays eggs and has 4-stages in its life-cycle. Q could be butterfly or mosquito. They lay eggs, and their life-cycle has 4-stages – egg, larva, pupa and adult. The pupa stage is the non-feeding stage.
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 5
Ans 3:
Class : Class 5
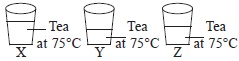
5I Think that there is right answer given and the right explaination given. As I am a student , ( Kendriya Vidyalaya ) a very very very very great mistake is there in the question image. It is written 75 degree but there should be just tea written there. If you say that the image is right, no problem but the answer should be AAAAA.Do you like the solution? this is written with the supervision and direction of my science teacher.
Ans 5:
Class : Class 6
The answer of this question should be A as the temperature is specified and same in all the glasses.
Ans 11:
Class : Class 5
the answer is A because all the three glasses that is A,B,C is at 75 degrees threfore option A is the right answer
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 4
Ans 1: (Master Answer)
Class : Class 1
B
Length of the day depends on the speed of rotation of a planet on its axis. The more is the speed the shorter is the length of the day.
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 6
Read the given passage and fill in the blanks by choosing an appropriate option.
Iron nails when new are grey and bright. When exposed to (i), they get covered with a (ii) coating. This process is called (iii) and it is a (iv) change.
| (i) | (ii) | (iii) | (iv) | |
| A | Moist air | Grey | Corrosion | Chemical, reversible |
| B | Air and water | Reddish brown | Rusting | Chemical, irreversible |
| C | Oxygen in air | Green | Rancidity | Physical, irreversible |
| D | Nitrogen in air | Reddish brown | Galvani-sation | Chemical, irreversible |