Subject :NSO Class : Class 5
Class : Class 7
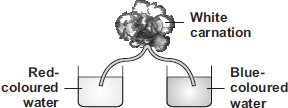
The stem absorbed the color along with the water, so some parts of the stem and the flower turned blue or red.
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Ans 4:
Class : Class 6
Ans is C. See PERSON in number positions:
16 05 18 19 15 14. Now keep the numbers in ascending order:
05 14 15 16 18 19:ENOPRS. Now OLYMPIAD in number positions:
15 12 25 13 16 09 01 04. Now keep the numbers in ascending order:
01 04 09 12 13 15 16 25:ADILMOPY.
Post Your Answer
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 4
Ans 3: (Master Answer)
Class : Class 1
The correct answer is A.
When food is taken in the mouth, it is chewed and gets mixed with saliva secreted in the mouth (R). Once swallowed, the food moves through the oesophagus into the stomach where churning and digestion of food with digestive juices occurs (Q). The semi-digested food then moves into the small intestine where complete digestion as well as absorption of nutrients occur (S). From the undigested food, the water is absorbed in the large intestine (P). After this, the undigested food is expelled through the anus in the form of faeces (T).
Ans 12:
Class : Class 3
The graph X-Axis is not describing digestive system it is describing four components of food which are carbohydrates, proteins, fats, and vitamins and minerals. So, vitamins and minerals are same before digestion and after digestion. answer is S.
Ans 18:
Class : Class 7
A is the correct answerWhen food is taken in the mouth, it is chewed and gets mixed with saliva secreted in the mouth (R). Once swallowed, the food moves through the oesophagus into the stomach where churning and digestion of food with digestive juices occurs (Q). The semi-digested food then moves into the small intestine where complete digestion as well as absorption of nutrients occur (S). From the undigested food, the water is absorbed in the large intestine (P). After this, the undigested food is expelled through the anus in the form of faeces (T).
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 2
Ans 1: (Master Answer)
Class : Class 1
Please visit our website- http://www.sofworld.org or call us at 0124-4951200 (Landline No), 9312680855, 9312680857 (Mobile:).
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 9