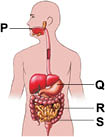
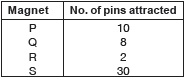
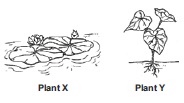
Subject :NSO Class : Class 5
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 1
In the given word grid:

(A) How many flesh eating animals are listed?
(B) How many plant eating animals are listed?
| (A) | (B) | |
| A | 2 | 3 |
| B | 2 | 4 |
| C | 3 | 1 |
| D | 5 | 4 |