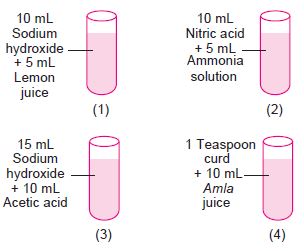
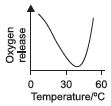
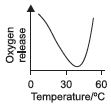
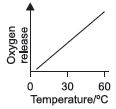
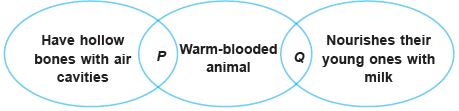
Subject :NSO Class : Class 7
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 8