Subject :NSO Class : Class 3
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 6
Match the column I with column II and select the
correct option from the given codes.
| Column I | Column II | ||
|---|---|---|---|
| (i) | 1 metre | (p) | 1 decimetre |
| (ii) | 1000 millimetres | (q) | 1000 metres |
| (iii) | 100 millimetres | (r) | 10 decimetres |
| (iv) | 1 kilometre | (s) | 10 metres |
| (v) | 1 decametre | (t) | 100 centimetres |
A(i)-(t); (ii)-(p), (iii)-(q), (iv)-(r), (v)-(s)
B(i)-(p); (ii)-(s), (iii)-(r), (iv)-(q), (v)-(t)
C(i)-(q); (ii)-(r), (iii)-(p), (iv)-(s), (v)-(t)
D(i)-(t); (ii)-(r), (iii)-(p), (iv)-(q), (v)-(s)
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 6

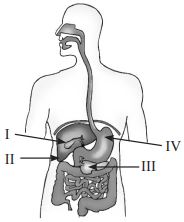
 , Butter
, Butter
 , Butter, Cheese
, Butter, Cheese
 , Bread
, Bread
 , Red meat
, Red meat