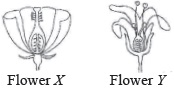
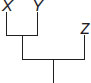
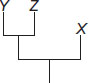
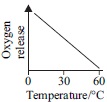
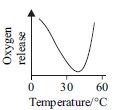
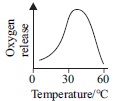
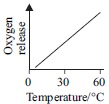
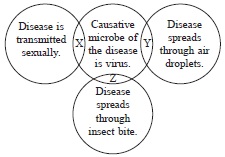
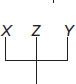Subject :NSO Class : Class 6
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 3
Match the columns and select the correct option.
| Column I | Column II |
| (A) It takes the shape of its container. | 1. Solid |
| (B) It has a fixed shape. | 2. Liquid |
| (C) If you freeze a liquid it will become this. | 3. Gas |
| (D) If you boil a liquid it will become this. |
A (A)-3, (B)-2, (C)-1, (D)-3
B (A)-2, (B)-1, (C)-1, (D)-3
C (A)-2, (B)-2, (C)-3, (D)-3
D (A)-1, (B)-2, (C)-1, (D)-3
Post Your Answer
Subject :NSO Class : Class 5