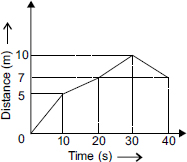
Post Your Answer
Post Your Answer
The bumpers and door handles of motor cars, taps etc. are coated with silvery layer of chromium because
(i) It does not easily get corroded.
(ii) It forms a hard layer which does not easily get scratched.
(iii) It tarnishes quickly.
(iv) It is cheap.
Which of the following options is correctly identifies true (T) or false (F)?
| (i) | (ii) | (iii) | (iv) | |
| A | F | F | T | T |
| B | T | T | F | F |
| C | F | T | F | T |
| D | T | T | F | T |
it is wrongshould be b
Ans 2:
Ans 4:
Ans 9:
Ans 10:
Post Your Answer
Post Your Answer
Post Your Answer
Post Your Answer
Ans 2: (Master Answer)
The correct answer is B
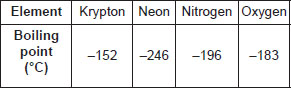
The gas having lowest boiling point i.e., highly volatile will be distilled out first and the gas having highest boiling point i.e., least volatile will be distilled out at the last. So, the correct order of gases distilling out is neon, nitrogen, oxygen, krypton.
Post Your Answer
Ans 2: (Master Answer)
The correct answer is B
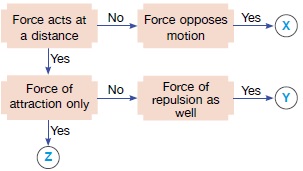
Here forces X, Y and Z are Friction, Magnetic force and Gravitational force. It is friction which opposes or slows down moving objects