Post Your Answer
Ans 1:
Ans 2: (Master Answer)
A tendril is a specialized stem, leaves or petiole with a threadlike shape that is used by climbing plants for support and attachment
Post Your Answer
Ans 1: (Master Answer)
The correct answer is D
Current carrying wire produces magnetic field, but direction of magnetic field on the given points X and Y are different.

This is found by right hand thumb rule. So magnetic compasses on X and Yexperience magnetic force of same magnitude but in opposite directions. Hence, the needles will deflect in opposite directions.
Post Your Answer
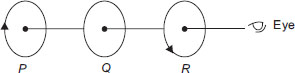
Ans 1: (Master Answer)
The correct answer is B
Coil P is moved with a uniform speed towards Q while R is static. Due to the current in P, magnetic field is from right to left. When P is moved towards Q, magnetic lines passing through Q (from right to left) will increase, i.e., magnetic flux passing through Q will increase. Therefore, current will be induced in Q. The induced current will have such a direction that it gives a magnetic field opposite to that passing through Q due to current in P. Thus, anticlockwise current will be induced in coil Q, opposite to the direction of current in coil P.
Post Your Answer
Post Your Answer
Ans 1: (Master Answer)
The atomic number and the atomic mass of iridium are 77 and 192.217 u ± 0.003 u respectively