Subject :NSO Class : Class 6
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Class : Class 10
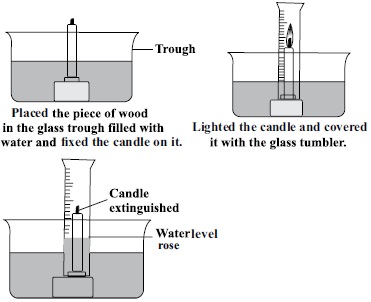
The correct option is D as of you actually perform it , you will see that water rises by 21% this is as combustion required oxygen which is about 21%in volume . Since it was exhausted , and space occupied air was lowered by 21%. , The water rose . This it proves
1) air has space
2) air has oxygen
Ans 2:
Class : Class 10
The correct option is D as of you actually perform it , you will see that water rises by 21% this is as combustion required oxygen which is about 21%in volume . Since it was exhausted , and space occupied air was lowered by 21%. , The water rose . This it proves
1) air has space
2) air has oxygen
Ans 3:
Class : Class 10
The correct option is D as of you actually perform it , you will see that water rises by 21% this is as combustion required oxygen which is about 21%in volume . Since it was exhausted , and space occupied air was lowered by 21%. , The water rose . This it proves
1) air has space
2) air has oxygen
Post Your Answer
Subject :NSO Class : Class 2
Post Your Answer
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7


 means
means  ,
,  means
means  and
and  means
means  , then which of the following can fly?
, then which of the following can fly?