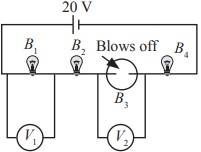
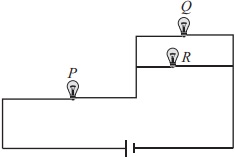
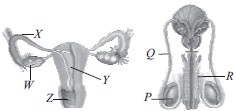
Subject :NSO Class : Class 7
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 6
Study the given flow chart and select the option that correctly identifies P, Q and R.
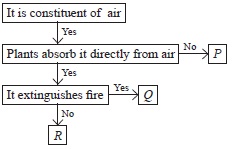
| P | Q | R | |
| A | Nitrogen | Oxygen | Carbon dioxide |
| B | Oxygen | Carbon dioxide |
Nitrogen |
| C | Nitrogen | Carbon dioxide |
Oxygen |
| D | Carbon dioxide |
Nitrogen | Oxygen |
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 4