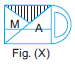
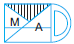
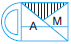
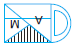
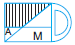
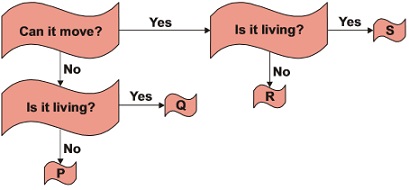
Subject :NSO Class : Class 4
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 1
Post Your Answer
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 8
Ans 4:
Class : Class 10
Density of oil = 0.80 g/cc = 800 kg/m3Density of water = 1 g/cc = 1000 kg/m3Pressure of water = Height will be 1 m half filledâ¹ hdg = 1mÃ1000kg/m3Ã10m/s2 = 10000 PaPressure by oil = hdg = 1 x 800 x 10 = 8000 PaTotal pressure = 8000Pa 10000Pa = 18000Pa = 1.8Ã104N/m2
Post Your Answer
Subject :NSO Class : Class 7
Ans 7:
Class : Class 8
silk smells of charred meat . This was the answer given even in the chapterwise question bank
Ans 8:
Class : Class 7
All options are wrong, it can't be A because cotton burns with smell of burning paper and similarly it can't be D because silk burns with an odour of charred meat.
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 6