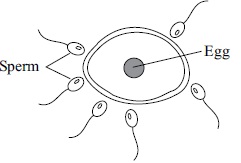
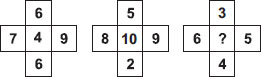
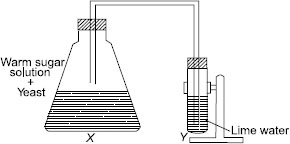
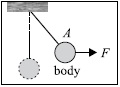
Subject :NSO Class : Class 8
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 3
Study the given food web and select the correct option for the following questions.
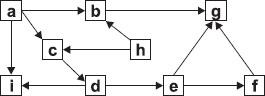
Which one of the following shows the correct grouping of the various organisms in the food web?
| Producer(s) | Herbivore(s) | Omnivore(s) | Carnivore(s) | |
| A | a, h | c | b | i, g, e, f, d |
| B | a, h | c, d | b | i, g, e, f |
| C | a, h | b, c | i, g | d, e, f |
| D | a, h | b, c | i | d, e, f, g |
I DONT UNDERSTAND
Post Your Answer
Subject :NSO Class : Class 8
Ans 3:
Class : Class 8
I think that option A and C should be correct. Since option A talks about fermentation occuring which could be true considering that yeast is put into warm sugar solution. Option C is also correct since we know that one of the end products of fermentation is CO2 and lime water turns milky white when it is combined with carbon-dioxide.
Ans 4:
Class : Class 8
Option C is the correct answer because yeast causes fermentation .Fermentation is an anaerobic process. In this process yeast converts sugar into alcohol and carbon dioxide. Lime water turns milky indicating the presence of carbon dioxide.
Ans 6:
Class : Class 8
answer cant be A since during fermentation of sugar alcohol is produced and not lactic acid
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 3
Ans 1:
Ans 6:
Class : Class 3
fgdgcbcbc vccdhdhdgvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvvbcbdfdhgdhvghvbvhfhhfudfdvbccjcccbcbcbdhdhssjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjjssssssssssssssssssssssssssssssssssss