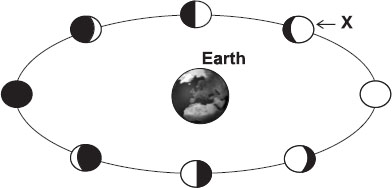
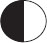
Subject :NSO Class : Class 8
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 4
Ans 2:
Class : Class 4
sdfghjklllllllllllllllllllllllllllllllllllllllllllllllkhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhhh5h5555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555555ddddddddddddddddddddddddddddddddddddddddddddddddddddddd
Ans 4:
Ans 9:
Class : Class 8
This is a dress worn by Maharstrian ladies. Maharasthra is not in the north India.It is part of Central India so, the correct answer should have been D.
Ans 10:
Class : Class 9
its not the same saree .Nauvari saree is 9 meters against the 6 meters north indian saree
Ans 17:
Class : Class 7
we realized the saree style is called Nawaree and its worn by a Maharashtrian lady. However its not NorthIndian its Western style
Post Your Answer
Subject :NSO Class : Class 4
Ans 5:
Class : Class 5
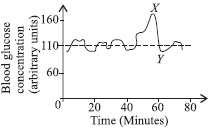
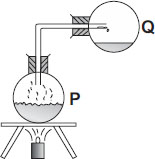
No, Ayva and my other friends who have a doubt... Remember that whenever any liquid while heating or boiling will not make its quantity decrease or increase. For Example -I heat 200ml of water until it started evaporating completely...it will not increase or decrease as I said earlier...it will remain 200ml only. That's why the answer is D.
Ans 10:
Class : Class 8
some vapour will condense in the tube before they reach flask q making it 350 ml. and is b
Post Your Answer
Subject :NSO Class : Class 3
Refer the given diagram of the human digestive system and answer the following question.
Which of the labelled parts
(i) contains digestive juices?
(ii) contains semi-solid wastes called faeces?
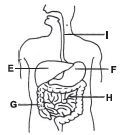
| (i) | (ii) | |
| (A) | E | H |
| (B) | F | G |
| (C) | F | H |
| (D) | I | G |
Post Your Answer
Subject :NSO Class : Class 6
Ans 3:
Class : Class 10
Silk can't be worn in monsoon and winter, because it is not waterproof and it does not trap heat.
Ans 5:
Class : Class 10
c should be the answer as silk is obtained from silkworm(insect) by harmingand wool,angora wool , etc are obtained without harming the mammal
Ans 11:
Class : Class 9
(D) is totally false . raincoat is made from plastic only which is a man made fiber .
Post Your Answer
Subject :NSO Class : Class 3