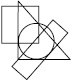
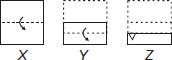
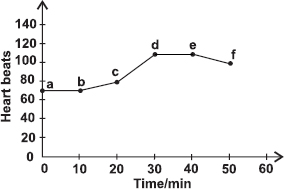
Subject :NSO Class : Class 8
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Match column I with column II and select the correct option from the codes given below.
| Column I (Salts) | Column II (Uses) |
| P. Baking soda | 1.In making fire-works |
| Q. Nitre | 2.As a fungicide |
| R. Blue vitriol | 3.In purification of water |
| S. Phitkari | 4.In fire extinguishers |
A P-3; Q-2; R-4; S-1
B P-4; Q-3; R-1; S-2
C P-4; Q-1; R-2; S-3
D P-2; Q-1; R-4; S-3
Post Your Answer
Subject :NSO Class : Class 5