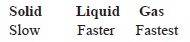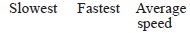Subject :NSO Class : Class 8
Subject :NSO Class : Class 8
Ans 2:
Class : Class 7
I'm not sure what the answer is in this one, as the correct answer and the solution are mismatched.
Ans 7:
Class : Class 8
The solution says that the answer is supposed to be d but according to the explanation given the answer seems to be a. Please explain.
Ans 8:
Class : Class 8
According to Faraday's right hand rule, electricity flowing from point A to B generates a magnetic field around the wire moving from right to left (above the wire), and left to right (below the wire). Due to the compass being above the wire (according to the question), the compass needle must be influenced by the magnetic field, such that it points towards the right; i.e. the needle points in a direction from left to right. Thus, I opine that option (A) is the correct answer, not option (D). As there is an electric current, there must be a magnetic field, and thus there must be a deflection in the compass, which excludes option (D) entirely.
Post Your Answer
Subject :NSO Class : Class 7
Ans 1:
Class : Class 8
Answer is option a as decomposition of dead matter of plant and animal by animals which gives nutrients to plants
Post Your Answer
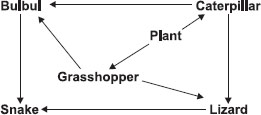
Subject :NSO Class : Class 3
Ans 1:
Class : Class 3
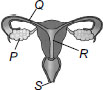
D - Because increase in plant also increases population of Caterpiler and Grasshopper, and decrease in snake may constant Bulbul population if plant did not increases.
Ans 6:
Class : Class 7
B first look which animals are connected to Bulbul with an arrow find their population then find population of other animals answer - B
Post Your Answer
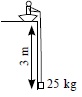
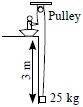
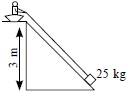
Subject :NSO Class : Class 5
Ans 2:
Class : Class 5
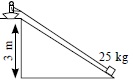
B because if we use a rope to pull it will take a lot of effort, if we take a small or large inclined plane it will also take more effort because the load will slip down but in pulley we have to only pull the rope
Ans 5:
Class : Class 10
Single pulley does not provide any mechanical advantage, it only changes the direction of force while an inclined plane provides mechanical advantage. This means the efforts required to life the same amount of load on an inclined plane is lesser than that by a single pulley. (Pulley provides mechanical advantage only if we use more than one pulley).Lesser the inclination more is mechanical advantage and lesser the effort. That means the correct answer should be 'D'
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 5
Ans 6:
Class : Class 6
Of the three mediums, sound waves travel the slowest through gases, faster through liquids, and fastest through solids. Sound waves need a medium to pass through. Liquid molecules are not packed as tightly as solids, and gases are very loosely packed. The spacing of the molecules enables sound to travel much faster through a solid than a liquid or a gas. Sound travels about four times faster and farther in water than it does in air. That means the right answer is Option D.i am the smartest guy in sofi have got once international rank 1in level 2
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 4