Subject :NSO Class : Class 9
Subject :NSO Class : Class 6
Ans 1:
Class : Class 7
W are roots which store food in them . X is a stem and onion is also a stem. Y are fruits of the plant and we call them as vegetables. Z are seeds but potatoes are stems , so they are not same.
Ans 3:
Class : Class 6
Wâ is the modified fleshy tap root of turnip which stores the reserve food material. âXâ represents garlic i.e. an underground stem modification. In both garlic and onion, fleshy scales are edible. âYâ is tomato, which is a fruit but is eaten as a vegetable. âZâ represents wheat grains, which are edible seeds but in potato stem is edible.
Ans 6:
Class : Class 9
I think its C . I searched the internet and it shows that tomato is a fruit eaten as a vegetable
Post Your Answer
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 3
Study the given flow chart carefully. Which one of the following options correctly describes the properties R, S and T in the chart?
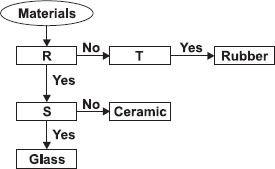
| R | S | T | |
| A | Allows light to pass through | Conducts electricity | Fragile |
| B | Fragile | Allows light to pass through | Flexible |
| C | Flexible | Conducts electricity | Hard |
| D | Conducts electricity | Fragile | Allows light to pass through |
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 3
Sandhya observed the Moon from 14th January to 28th January. She noted that Full Moon appeared on 18th January. Which of the following options correctly shows the phases of the Moon, Sandhya observed on 14th January and 28th January respectively?
| 14th January | 28th January | 14th January | 28th January |
A

B

C

D

Post Your Answer
Subject :NSO Class : Class 6
Ans 5:
Class : Class 9
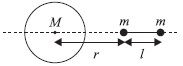
Usually leaves having reticulate venation have tap roots and leaves having parallel venation have fibrous root, hence the answer is c because tap roots provide better anchorage than fibrous root
Post Your Answer
Subject :NSO Class : Class 9
Post Your Answer
Subject :NSO Class : Class 3
Ans 7:
Class : Class 8
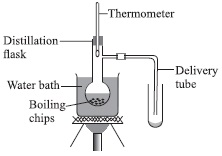
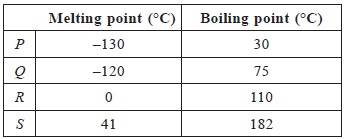
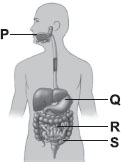
Answer: AExplanation: Food is masticated with teeth and partially digested by the action salivary juices in the mouth (P).
Post Your Answer
Subject :NSO Class : Class 6