Subject :NSO Class : Class 7
Class : Class 7
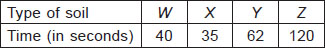
All the given factors are required for a fair test ; So option D would be the correct answer
Class : Class 8
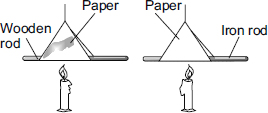
shape of dish also should be included. Amount of water poured doesnt matter as long as you are collecting equal volume in all dishes. Hence similar dishes is important.
Subject :NSO Class : Class 6
Ans 6:
Class : Class 9
C,because- it is a chemical change. after baking we can break it into smaller particles but it won't form the same clay, with same properties again
Ans 7:
Class : Class 6
on heating, the clay pot doesn't turn into stone,right?it doesn't change composition once heated , it is still clay ! answer is "A"
Ans 9:
Class : Class 7
A is correct because it just turns hard but it is still clay only.And also chemical change doesn't mean that it is irreversible,Chemical change means that the identity of the substance we started with is lost.Here the identity is not lost.So A is correct.
Ans 12:
Class : Class 6
Yes ,Because chemical means change in the properties but there is no change .The answer is A
Ans 13:
Class : Class 6
IF WE SHARP A PENCIL IT IS A PHYSICAL IRREVERSIBLE CHANGE .SO,IF WE HEAT A POT IS PHYSICAL IRREVERSIBLE CHANGE. BUT IT COULD HAPPEN THAT THE COMPOSITION OF CLAY MIGHT CHANE BECAUSE OF HEATING AS THE MOLECULES STICK TO EACH OTHER
Ans 15:
Class : Class 6
C because we can get clay back from a wet pot by smashing it,but once it heats up, it dries the clay and makes it stone hard.
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 7
Saurabh burned two different fibres X and Y in order to identify them. X smelt like burning paper while Y smelt like burning hair. Which of the following options correctly identifies the two fibres?
| X | Y | |
| (A) | Wool | Silk |
| (B) | Cotton | Rayon |
| (C) | Cotton | Silk |
| (D) | Silk | Wool |