Subject :NSO Class : Class 5
Class : Class 9
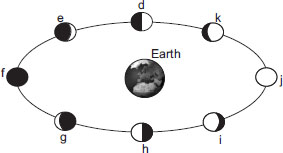
It is a fact that the highest tides occur when the moon is full or new. In the diagram, the positions F and J are the full moons and new moons, so the correct answer should be B
Class : Class 10
Look at the diagram.........the faces of moon closest to the earth are D and H, hence the suitable solution is A
Class : Class 9
just because we can see the whole moon during a full moon dosen't mean that on that day there will be the greatest tides as the moon dosen't cut itself into half on 1st and 3rd quarter. So, the answer must depend on the distance of the moon from the earth as the gravitational pull of the moon will have more effect when it is closer to earth and will raise bigger tides. The moon is closest to earth at phases D and H. So, clearly the answer should be A
Class : Class 6
The answer is (a) because when the full moon and new moon are there then the sun, earth and moon are aligned and the sun is also pulling at the sea forming big tides.
Subject :NSO Class : Class 6
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 7
Ans 6:
Class : Class 9
Both the science and geography textbooks of NCERT state that there is low pressure at 60 degree North and South also. So, the answer is D.
Ans 10:
Class : Class 8
Answer should be D only . There is low pressure at the equator and at 60 degree north and south pole
Ans 12:
Class : Class 6
The answer is D as low pressure exists in both equator and 60 degree north and south.
Ans 16:
Class : Class 9
if you are showing this answer wrong in Olympiad training paper and if we get the same question in examination which answer will be accepted as correct?
Ans 24:
Class : Class 6
yes, the correct answer is D since there are two belts of low pressure on the earth. on the equator (due to high temperature) and between the two belts of high pressure (60 degrees North and South. ). A cannot be the correct answer.
Post Your Answer
Subject :NSO Class : Class 8
Ans 5:
Class : Class 8
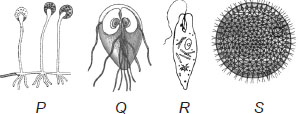
A protozoan or alga isn't considered as a connecting link between living and non-living things. A virus is considered so, which is Diagram S. Admin from Revisewise Noida is kindly requested to explain how A is going to be the correct answer.
Ans 11:
Post Your Answer
Subject :NSO Class : Class 5
Ans 2:
Class : Class 7
Answer is D and nothing ! nothing ! nothing !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!SOF is wrong ! wrong ! wrong !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
Ans 3:
Class : Class 1
Ans 5 is right and so are the answers SOF is wrong. And ans 1 I think u need to tell that to some elder
Ans 4:
Class : Class 5
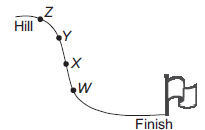
Well, isn't Option A the place where it slows down 'cause that's the point where the low slope starts so I thought it would be point X (Option B)
Ans 7:
Class : Class 9
Guys - NOT SOF but you all are wrong. I reviewed the question and saw it was written - A ball has started from a hill. Where will it be the fastest or where is the most kinetic energy so it is A. point W. If it was from which point should it start to reach the flag the fastest it would be D
Ans 10:
Post Your Answer
Subject :NSO Class : Class 6
Ans 1:
Class : Class 7
Stop talking such bad words. We are doing this because too many people are getting full marks so we made it have no options so there are fewer people getting full marks
Ans 4:
Class : Class 7
No options were given! Sof, please please fix this problem.You have done other questions like showing wrong answer.But this was not fair!! At least give options
Ans 7:
Class : Class 6
OPTIONS ARE NOT GIVEN!!!!!!!!!!!!!!! WHY ARE U DOING THIS??????EVEN AFTER MANY REPORTING ABOUT IT
Ans 11:
Class : Class 9
SOF after 31 (and add me then 32) PEOPLE HAVE REPORTED YOU HAVE NOT FIXED THIS ISSUE! MAY I KNOW WHY YOU HAVE DONE SO????????????? THIS IS WRONG AND I'm GONNA REPORT TO sofworld.org. I think friends everyone should do this. Maybe they don't want us to give the answer. SO MANY MISTAKES BEING DONE BY SOF IN THE QUESTIONS! and MASTER ANSWER PLEASE RECTIFY THIS ERROR. Because of you our 1 mark is gone, SOF. Plz rectify!
Ans 15:
Class : Class 9
Dear SOF,I just like many students was not able to see the options. I have to choose a random answer(which like expected, was the wrong answer) as suggested by norom. Kindly fix this problem.
Ans 20:
Class : Class 9
Oh, JUST BECAUSE PEOPLE ARE GETTING BETTER EVERYDAY BY PRACTICING, YOU DONâT WANT THEM TO! SO WHAT IF THEY GET FULL MARKS, ATLEAST WE ARE PROGRESSING, YOU SOF PEOPLE ARE FOOLS
Ans 27:
Class : Class 9
SOF then what is the advantage. Then will you give question paper also like this. I will call this favourism to those who are getting these options
Ans 33:
Class : Class 6
not a single option was given.never mind if its given wrong. you just need to understand and try to solve it.
Ans 35:
Class : Class 9
No Option given. I will never buy this package again. Selfish for money. Not rectifying this for more than 2 years. Worst Sof
Post Your Answer
Subject :NSO Class : Class 7
Ans 1:
Class : Class 8
Answer is B
A : Climber moves upwards along with the support of other plant or wall.
B : Runner are stems that form adventitious roots at the nodes and new plants from the buds.
C : Tendril is a specialized stem,leaf or petiolr with a thread like shape used for support by climbing plants.
D : Bulbil is a bulb like structure in axil of a leaf which may fall to form a new plant.
Post Your Answer
Subject :NSO Class : Class 5