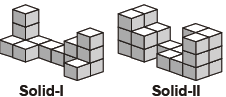
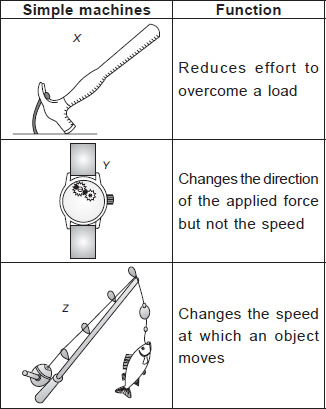
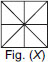
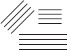
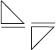
Subject :NSO Class : Class 3
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 5
Ans 4:
Ans 6:
Class : Class 5
C is the answerIf you open synopsis in this chapter,it is clearly written that a compass works with the help of earth's magnetic field
Post Your Answer
Subject :NSO Class : Class 7
Ans 6:
Class : Class 3
Turmeric indicator remains yellow in acidic (vinegar) and neutral solutions (common salt solution) and turns red in basic solution (soap solution and baking soda).
Ans 15:
Class : Class 7
They have not written: Both B and C in the D option. Whereas, B is important in the D option.
Post Your Answer
Subject :NSO Class : Class 5
Ans 1:
Class : Class 1
The correct Ans is B and I did B but SOF is telling me I did C. Impossible and I am telling the truth I swear.
Ans 6:
Class : Class 5
From Athiya Mahapatra:
option)c
As the Upper layer of Earth is called Crust. The liquid which flows outside a volcano is called lava and Richter Scale is used to measure intensity of earthquakes.
Ans 8:
Ans 10:
Ans 12:
Class : Class 6
Volcano is an opening on the surface of Earthâs crust. There are three types of volcanoes: (i) Active volcanoes are those which are currently erupting or have erupted in the last 10,000 years. (ii) The dormant volcanoes are in a state of sleep. This means that they had erupted in the past but can erupt again, however, at the moment they are asleep. (iii) Extinct volcanoes are those that have been inactive for thousands of years and are not expected to erupt again. The liquid that comes out of a volcano is called lava. Richter scale measures magnitude of earthquakes and was developed by Charles Richter in 1935.
Ans 13:
Class : Class 10
I feel the (B) should be the correct answer, so please explain why (C) is the correct one.
Post Your Answer
Subject :NSO Class : Class 5
Ans 1:
Class : Class 5
First of all, fish, crocodile and snakes, all these three are egg laying animals. Secondly, all of them are cold blooded animals. And thirdly, all of them are covered with scales. And therefore, statements 1, 2 & 3 are correct. That is why option C is correct.
Post Your Answer
Subject :NSO Class : Class 5
Ans 5:
Class : Class 6
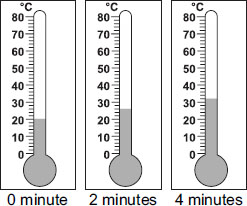
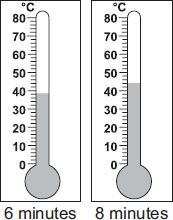
Ans is C because 0 minutes was 20 degrees celsius. Then, we were adding 6 after 2, 4, 6 minutes and so on.
1 is half of 2, and 1+2=3. So we should take normally 26 degrees celsius+(Half of 6) degrees celsius=26 degrees celsius+3 degrees celsius=29 degrees celsius.
Post Your Answer
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 8
Post Your Answer
Subject :NSO Class : Class 6