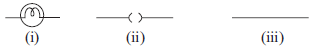Subject :NSO Class : Class 8
Subject :NSO Class : Class 5
Post Your Answer
Subject :NSO Class : Class 4
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 5
Ans 8:
Class : Class 3
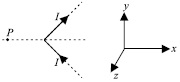
All metal expands on heating. Here metal Y expands more which leads to curling of both X and Y. B is the correct option.
Ans 9:
Class : Class 4
ans should be D as if metal x contracts and metal y expands then the 2 metals will bend inward an as an ellusion we see that y is longer than x
Ans 15:
Class : Class 5
as if we will contract metal x so it is not neccessery that y will contract if we contract metal y x has to be conyracted with it ans should be a
Ans 18:
Class : Class 1
The answer is B because the question says that he HEATED both the metals (most metals expand on heating) and Y has expanded more than X
Ans 20:
Class : Class 4
iron-nickel alloy notable for its lack of expansion or contraction with temperature changes. hence all metals do NOT expand
Ans 22:
Class : Class 7
Sumit Singhal , this is a bimetallic metal . Here , the two metals cannot go away from each other . So here , if y expands than x will also expand , and if x expands than y will also expand . I am getting confusion between A and B .
Ans 24:
Class : Class 9
B because if Y expands and X contracts, X will be a lot smaller than Y. Here, both are almost the same size.
Post Your Answer
Subject :NSO Class : Class 8
Ans 1:
Class : Class 9
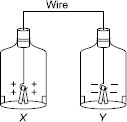
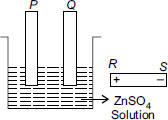
I will go mad with the number of wrong answers in this website.. answer should be a. For example when a spoon has to be electroplated with copper, copper is ALWAYS ON ANODE and the spoon is ALWAYS ON CATHODE. Why different logic is used here?
Ans 9:
Class : Class 6
answer should be A, as hydrogen is not released at cathode if the other cation is Zinc or below in the electrochemical series
Ans 14:
Class : Class 7
i do feel that correct answer is A.as P has to be electroplated ,it must be on cathode(-ve).so,correct is =A
Ans 18:
Class : Class 10
In reactivity series hydrogen is below zinc and is less reactive than zinc. As hydrogen below zinc therefore will reduce more and earlier than zinc , hence will go to cathode and zinc will reduced or deposit at anode. therefore P will be connected to R and Q to S.hence ans is :C
Post Your Answer
Subject :NSO Class : Class 3
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 7
Post Your Answer
Subject :NSO Class : Class 5
Ans 1:
Class : Class 9
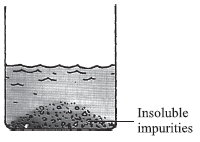
it needs to be sedimentation and decantation because the insoluble material present in the liquid is quite heavy
you only perform filtration when the particles float over the surface of the water
Ans 2:
Class : Class 5
Ya, filtration can also remove insoluble impurities but in this case sedimentation and decanation is more appropriate answer.
Ans 4:
Class : Class 5
sedimentation and decantation is the answer .
but filtration can not come because we can see in the picture itself that the particles are big ,that's why sedimentation and decantation .
if the particles are small but yet insoluble , then only it is filtration .
I hope that you understood.

 . It is common in Kashmir and
. It is common in Kashmir and