A hot liquid is carefullypoured into a beaker. The graph shows how its temperature changes as it cools towards room temperature.
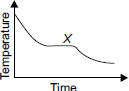
Which process(es) is/are taking place at region X ?
A Boiling and evaporation
B Condensation only
C Evaporation only
D Solidification and evaporation
All the latent heat of vaporization is used for condensation. So at X the T is constant
Ans 1:
Edit Post
kashvi
from
Amity, noida
Class : Class 7
Three states - gas , liquid & solid
When hot water temp is going down it will be ultimate become solid ie ice
But before becoming ice temp will stagnant for some time where in both states liquid & solid co-exist==> some part of liquid is in ice form.
X is the same state where in solid and liquid will co-exist. Hence option 4 is correct(solidification& evaporation)
Note: Could not attach gif .It would have made it more clear.
Ans 7:
Edit Post
Class : Class 7
I think B is the correct answer because there is no solidification taking place or else the water would have turned into ice. plus no solidification can take place when water is at room temperature.
Ans 8:
Edit Post
Ans 9:
Edit Post
Class : Class 8
at 4 degree celsius water starts expanding and 'x' shows the same the room temperature might have been near or equal to the freezing point of water(eg room temperature of regions like arctic and antarctic)
Ans 10:
Edit Post
Class : Class 8
It is cooling towards room temperature so it does not solidify since the freezing point is below room temperature.
Ans 11:
Edit Post
Ans 12:
Edit Post
Class : Class 10
i think there is no option correct in this ques. no evaporation and boiling is taking place as it is cooling down towards the room temperature. also no condensation is taking place as in the ques it is written a HOT liquid is being transferred to a beaker. as well as solidification may also not take place because room temp is above 0 degree centigrate. SOF PLEASE SEE TO IT THAT THIS TYPE OF WRONGG QUESTIONS AND SOLUTIONS ARE NOT POSTED.
Ans 13:
Edit Post
Ans 15:
Edit Post
Ans 17:
Edit Post
Class : Class 7
There is no proper solution for this question which will make it more confusing as everyone will have different opinions in this question.