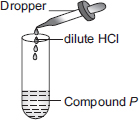
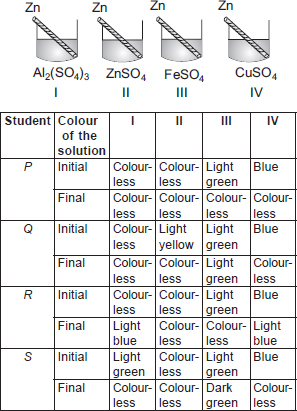
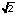Subject :NSO Class : Class 10
Class : Class 10
B) II,III,IV
CARBON can reduce oxides of metals lower in the activity series.
K, Ca and Mg are high in the activity series so they are very reactive so they can not be found in free state easily.
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10
Ans 1: (Master Answer)
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10
Ans 1:
Class : Class 10
It is because diamonds contain no free electron and hence do not conduct electricity.
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10
Post Your Answer
Subject :NSO Class : Class 10