The diagram shows a beaker containing a solution of zinc sulphate and two carbon electrodes. A battery is placed next to it. In order that the electrode P be plated with zinc,
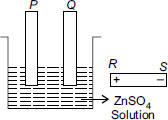
A P must be connected to S and Q to R
B P must be connected to Q and S to R
C P must be connected to R and Q to S
D P and Q must be connected to R.
The answer would be a as znso4 wold breakdown into zn 2and so42-ions .the zn 2 would be attracted towards cathode(negative terminal) so p would be attached to negative terminal and therefore the answer is a.
Ans 1:
Edit Post
Ans 6:
Edit Post
Ans 7:
Edit Post
Class : Class 6
answer should be A, as hydrogen is not released at cathode if the other cation is Zinc or below in the electrochemical series
Ans 12:
Edit Post
Ans 13:
Edit Post
Class : Class 7
i do feel that correct answer is A.as P has to be electroplated ,it must be on cathode(-ve).so,correct is =A
Ans 14:
Edit Post
Class : Class 9
I will go mad with the number of wrong answers in this website.. answer should be a. For example when a spoon has to be electroplated with copper, copper is ALWAYS ON ANODE and the spoon is ALWAYS ON CATHODE. Why different logic is used here?
Ans 16:
Edit Post
Class : Class 10
In reactivity series hydrogen is below zinc and is less reactive than zinc. As hydrogen below zinc therefore will reduce more and earlier than zinc , hence will go to cathode and zinc will reduced or deposit at anode. therefore P will be connected to R and Q to S.hence ans is :C