Observe the given figure carefully and choose the correct statement.
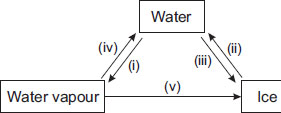
A Processes (iii) and (iv) involve absorption of heat energy.
B Process (v) involves condensation and freezing.
C Processes (i) and (iii) evolve heat energy.
D Process (v) absorbs a lot of heat energy.
How in processes v condensation came?
Ans 4:
Edit Post
Ans 5:
Edit Post
Class : Class 6
We can express process v as deposition and how did condensation and freezing get involved
Ans 6:
Edit Post
Ans 7:
Edit Post
Class : Class 7
Guys you are wrong process name is deposition but you need to cool it to water then freeze it
Ans 8:
Edit Post
Ans 19:
Edit Post
Class : Class 9
Answer is B because to turn Water vapour into ice you need Deposition but water vapour cannot be deposited that is why you first need to turn the water vapour to water by condensation and then to convert water to ice you need freezing . Hence answer B is correct