Observe the two figures given below. In figure I, a solution of sugar in water is heated and in figure II, only sugar is being heated. What is the correct sequence of changes taking place?
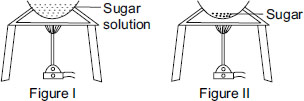
A In figure I, physical change is taking place while in figure II a chemical change is taking place.
B In figure I, a chemical change is taking place while in figure II a physical change is taking place.
C In both figures, physical changes are taking place.
D In both figures, chemical changes are taking place.
when water is added to water it undergoes a chemical change and not a physical change
Ans 1: (Master Answer)
Edit Post
Admin
from
revisewise, Noida
Class : Class 1
In figure I, evaporation of water from sugar solution is a physical change because there is only change in state of matter from liquid (sugar solution) to solid state (crystals of sugar). In figure II, when sugar is heated, it turns black due to the formation of a new substance and it cannot be reversed. Therefore, it is a chemical change.
Ans 8:
Edit Post
Class : Class 10
In figure II, when sugar is heated, it turns black due to the formation of a new substance and it cannot be reversed. Therefore, it is a chemical change.thats the reason