A copper vessel exposed to moist air for a long time is shown in figure I and sulphur powder is burnt in oxygen as shown in figure II.
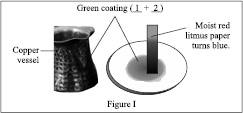

1, 2, 3 and 4 are respectively
ACuO, CO2, SO2, H2SO3
BCuCO3, Cu(OH)2, H2SO3, SO2
CCu, CuO, H2S, H2SO4
DCu(OH)2, CuCO3, SO2, H2SO3
Red litmus turns blue in a basic or alkaline solution. And red litmus turns blue in a acidic solution.(Please explain this.Copy pasted from the solution.)