Subject :NSO Class : Class 9
The given figure shows the set-up to study the reaction between gas X and copper (II) oxide :
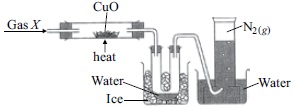
Which of the following statements are correct?
[Given : Atomic mass of N = 14 u, H = 1 u, O = 16 u,
C = 12 u]
I. Gas X is a compound of two elements, nitrogen and hydrogen.
II. The number of atoms present in 11.2 L of N2 is 6.023 × 1023.
III. 1 mole of H2O contains 1 mole of oxygen molecules and 2 moles of hydrogen atoms.
IV. N2 gas being soluble in water is collected by upward displacement of water.
AI and II only
BI and III only
CII and IV only
DIII and IV only


