Position of five elements P, Q, R, S and T is shown on the simplified form of the periodic table.
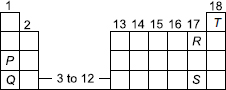
The element which has maximum tendency to lose electron, the element which has maximum tendency to gain electrons and the element which has no tendency to gain or lose electrons are respectively
A P, R and S
B S, R and T
C Q, S and P
D Q, R and T.
the correct answer for this question is option D.This is because Qhas more metallic property as compared to S,thus it more electropositive in nature .But the answer given for this question is B.I request you to revise the answer key
Ans 6:
Edit Post
Class : Class 10
the answer for this question is option D,since Q is more electropositive than s and thus will lose electrons easily .But in the answer key it is given as optionB.
Ans 7:
Edit Post
Class : Class 6
Q is a metal and S is a non metal, and therefore Q has a greater chance at losing electrons. The correct option is D.