Shaheen, a class 10 student studied the speed of the given reaction under different conditions.
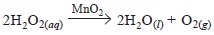
In experiment I, she added 1 g of MnO2 (catalyst) to
75 cm3 of 0.4 mol/L H2O2 solution at 30°C
In experiment II, she added 1 g of MnO2 to 100 cm3 of 'X' mol/L H2O2 solution at 'T' °(C)
She recorded her observations and plotted a graph as shown in the given figure.
The values of X and T in experiment II could be
respectively
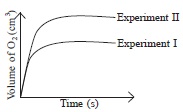
A 0.30 and 35
B 0.40 and 30
C 0.25 and 30
D 0.30 and 30.
How to find this?