Refer to the given graph representing changes in the states of water with temperature.
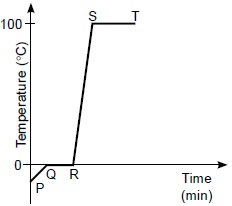
Based on the given graph which of the following statements are correct?
(i) Heat is gained from P to T.
(ii) There are different states of water along QR.
(iii) Vaporisation occurs at P to Q.
A(i) and (ii) only
B(ii) only
C(ii) and (iii) only
D(i), (ii) and (iii)
answer keys appear to be wrong