A metallic piece (X) is dropped into copper sulphate solution. After some time, the blue colour of the solution changes to green. The change which has occurred is
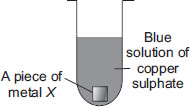
A Physical and reversible change
B Physical and irreversible change
C Chemical and reversible change
D Chemical and irreversible change.
due to the presence of copper in the metal the copper oxidzes faster an turn the solution green this same is done for extracting silver from aqua regia or royal water and the copper also can get crystalized
Ans 2:
Edit Post
SKIyer
from
CNS, Mumbai
Class : Class 6
No, missed it by some the properties of the metal mix with the solution. So according to me it is D which is the right answer
Ans 4:
Edit Post
Arnesh
from
dps, delhi
Class : Class 6
The answer is D as even if we move the metal the colour of the solution won't change.
Ans 6:
Edit Post
Ans 7:
Edit Post
Class : Class 7
guys logically if we take a protein sample and then after mixing it if we extract it the color gets reversed something with metal
Ans 9:
Edit Post
Class : Class 8
If we remove the metallic piece the solution's color won't change.So,Therefore it is an irreversable change and as it has been caused by Chemical Reaction of Metal and Copper Sulphate.So,Answer is D