In an experiment, hydrochloric acid was reacted with calcium carbonate at room temperature and pressure.
If 80 cm3 of gas X was produced then, the number of molecules and mass of gas X given off are respectively
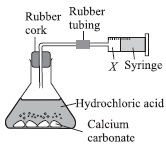
A 3.33 × 10-3, 0.5 g
B 2.15 × 1021, 0.157 g
C 6 × 1023, 7.5 g
D 4 × 1021, 1.5 g
is there anyone who can explain this question's solution