Study the given figure carefully and select the incorrect statement(s).
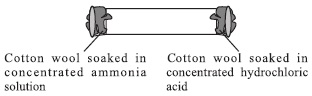
I. A white ring is formed which is closer to the
hydrochloric acid end of the tube because hydrogen
chloride fumes diffuse faster than ammonia.
II. Ratio of number of atoms of ammonia to that
of hydrogen chloride which combine to form
3.6138 × 1024 atoms of ammonium chloride is 1 : 3.
III. 3.4 g of ammonia combines with 7.3 g of
hydrogen chloride to form 10.7 g of ammonium
chloride.
IV. If temperature is increased, white ring will be
formed exactly in the middle of the tube.
(Given : Atomic mass of H = 1 u, N = 14 u, Cl = 35.5 u)
A II and IV only
B II and III only
C I, II and IV only
D I only
How to solve this