Four different-sized beakers are filled with water up to the same height. The temperature of the water is same in all four beakers. If we put 100 gram of ice
in each beaker, what effect will occur?
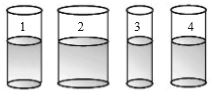
A The temperature of water in beaker 2 will change
the most.
B The ice will melt fastest in the beaker 3.
C The temperature changes will be same in all four
beakers.
D The temperature of the water in beaker 3 will
change the most.
how?