The boiling points of a few gases found in air are given below :
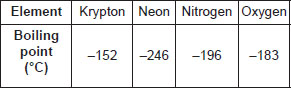
If liquid mixture is fractionally distilled, the order of gases distilling out is
A Krypton, Neon, Nitrogen, Oxygen
B Neon, Nitrogen, Oxygen, Krypton
C Nitrogen, Neon, Oxygen, Krypton
D Oxygen, Neon, Nitrogen, Krypton.