VV Souri
from
LITTLE FLOWER SCHOOL , UPPAL HYDERABAD
Subject :NSO Class : Class 7
A hot liquid is carefullypoured into a beaker. The graph shows how its temperature changes as it cools towards room temperature.
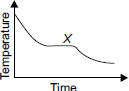
Which process(es) is/are taking place at region X ?
A Boiling and evaporation
B Condensation only
C Evaporation only
D Solidification and evaporation
All the latent heat of vaporization is used for condensation. So at X the T is constant
Ans 1:
Edit Post
Ans 3:
Edit Post
SOUVAGYA SAHA
from
De Nobili School, DHANBAD
Class : Class 9
i think there is no option correct in this ques. no evaporation and boiling is taking place as it is cooling down towards the room temperature. also no condensation is taking place as in the ques it is written a HOT liquid is being transferred to a beaker. as well as solidification may also not take place because room temp is above 0 degree centigrate. SOF PLEASE SEE TO IT THAT THIS TYPE OF WRONGG QUESTIONS AND SOLUTIONS ARE NOT POSTED.
Ans 4:
Edit Post
Pisal Dhruv
from
Veer Bhagat Singh International School, Mumbai
Class : Class 7
i think b is the correct answer
Ans 5:
Edit Post
Ans 6:
Edit Post
Ans 7:
Edit Post
Debarka Das Gupta
from
Silchar Don Bosco School, Silchar
Class : Class 7
i think b is the correct answer
Ans 8:
Edit Post
Ans 9:
Edit Post
Hritvi
from
Podar International School, Mumbai
Class : Class 7
I think B is the correct answer because there is no solidification taking place or else the water would have turned into ice. plus no solidification can take place when water is at room temperature.
Ans 10:
Edit Post
kashvi
from
Amity, noida
Class : Class 7
Three states - gas , liquid & solid
When hot water temp is going down it will be ultimate become solid ie ice
But before becoming ice temp will stagnant for some time where in both states liquid & solid co-exist==> some part of liquid is in ice form.
X is the same state where in solid and liquid will co-exist. Hence option 4 is correct(solidification& evaporation)
Note: Could not attach gif .It would have made it more clear.
Ans 11:
Edit Post
Ans 12:
Edit Post
Ans 13:
Edit Post
Aditya Sai Vemparala
from
Amanora school, Pune
Class : Class 10
Also freezing and melting point of water is 0 degrees celsius
Ans 14:
Edit Post
Hritvi
from
Podar International School, Mumbai
Class : Class 7
There is no proper solution for this question which will make it more confusing as everyone will have different opinions in this question.
Ans 15:
Edit Post
Sanidhya Kapoor
from
Shikshantar, Gurugram
Class : Class 8
It is cooling towards room temperature so it does not solidify since the freezing point is below room temperature.
Ans 16:
Edit Post
Aditya Sai Vemparala
from
Amanora school, Pune
Class : Class 10
It cools towards room temperature so it does not freeze
Ans 18:
Edit Post
arjun
from
the bishops school camp , pune
Class : Class 8
at 4 degree celsius water starts expanding and 'x' shows the same the room temperature might have been near or equal to the freezing point of water(eg room temperature of regions like arctic and antarctic)
Ans 19:
Edit Post
JANYA CHANGELA
from
UDGAM SCHOOL FOR CHILDREN , Ahmedabad
Class : Class 8
I THINK THAT B IS ONLY THE CORRECT ANSWER


